
Liaoning Xinda Talc Group Co., Ltd. - Cao Xinyu
Abstract
This article focuses on the purification of talc solid waste, using low-grade talc solid waste provided by Liaoning Xinda Talc Group Co., Ltd. as the research object, demonstrating the group's scientific research enthusiasm and innovative spirit in the field of efficient utilization of talc resources. The experiment used talc powder with a talc content of 31%, D50 of 800 mesh, 10.5% dolomite, and 58% magnesite as experimental samples, and conducted acid leaching experiments using 20% concentration hydrochloric acid. The experimental system studied the effects of acid leaching time, temperature, solid-liquid ratio and other parameters on the removal efficiency of dolomite and magnesite, and combined with XRD, SEM and other analytical methods, revealed the mechanism of acid leaching purification. The results showed that under the optimal process conditions (acid leaching time of 4 hours, temperature of 80 ℃, solid-liquid ratio of 1:15), the dissolution rates of dolomite and magnesite reached 98.5% and 99.1%, respectively, and the purity of talc was significantly improved. This study provides a scientific basis for the resource utilization of talc solid waste.
Keywords: talc solid waste; Acid leaching purification; Hydrochloric acid; process optimization
1. Introduction
As an important non-metallic mineral, talc (Mg3Si4O10 (OH) 2) is widely used in coatings, plastics, papermaking, ceramics and other fields due to its unique layered structure, lubricity, fire resistance and chemical stability. However, natural talc minerals are often accompanied by impurities such as dolomite and magnesite, resulting in insufficient purity and limiting their high-end applications. Although traditional physical mineral processing methods such as flotation and magnetic separation can partially remove impurities, their effectiveness in removing fine particle level impurities is limited, and there are problems with complex processes and high costs.
As a chemical purification technique, acid leaching achieves selective dissolution through the chemical reaction between acid solution and impurity minerals. It has the advantages of easy operation, high reaction rate, and thorough removal of impurities. This article takes low-grade talc solid waste from a certain mine as the research object, and uses 20% concentration hydrochloric acid for acid leaching experiments to systematically explore the removal effect of acid leaching process on dolomite and magnesite, aiming to optimize process parameters, improve talc purity, and provide theoretical basis for the resource utilization of talc solid waste.
2. Experimental materials and methods
2.1 Experimental raw materials
The experimental raw material is talc tailings provided by Liaoning Xinda Talc Group Co., Ltd. After X-ray fluorescence spectroscopy (XRF) analysis, its main components are shown in Table 1:
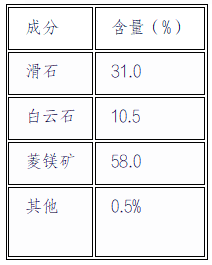
The raw material has a particle size of D50 and 800 mesh, with a specific surface area of 8.2 m ²/g, and is in the form of layered aggregates.
2.2 Experimental reagents and equipment
Reagent:
Hydrochloric acid (analytical grade, concentration 37%), sodium hydroxide (analytical grade), deionized water.
Equipment:
Constant temperature water bath (DF-101S), magnetic stirrer (85-2A), electronic balance (FA2004), centrifuge (TDL-5A), pH meter (PHS-3C), X-ray diffractometer (XRD, D8 Advance), scanning electron microscope (SEM, Quanta 250), laser particle size analyzer (Mastersizer 3000).
2.3 Experimental Methods
Acid leaching experiment:
Weigh 50g of talc solid waste sample, add 20% hydrochloric acid solution at a solid-liquid ratio of 1:5-1:30g/mL, and place it in a constant temperature water bath. Stir the reaction at 100-500rpm for 1-8 hours at 25-90 ℃. After the reaction is complete, the solid-liquid phase is separated by centrifugation, and the solid is washed with deionized water until neutral. After drying, the components are analyzed.
Determination of dissolution rate of dolomite and magnesite:
The concentration of Mg ² ⁺ in the acid leaching solution was determined by ICP-OES, and the dissolution rate was calculated by combining the stoichiometric relationship between dolomite (CaMg (CO3) 2) and magnesite (MgCO3) in the raw material. The formula is:
Dissolution rate (%)=m ore x wMgC liquid x V liquid x 100%
Among them, solution C represents the concentration of Mg ² ⁺ in the acid leaching solution (g/L), solution V represents the volume of the acid leaching solution (L), m ore represents the mass of the raw material (g), and wMg represents the mass fraction of MgO in the raw material (%).
Purity analysis of talc:
Using XRF to determine the talc content in solid products, combined with the law of conservation of mass to calculate the purity improvement rate, the formula is:
Purity (%)=m talc/m total x 100%
Among them, m talc is the mass of talc in the solid product (g), and m total is the total mass of the solid product (g).
Results and Discussion
Optimization of acid leaching process parameters
1. The influence of acid leaching time
The effect of acid leaching time on impurity removal efficiency. Within 1-4 hours, the dissolution rate of magnesite significantly increases with time and tends to stabilize after 4 hours; The dissolution rate of dolomite reaches over 90% after 2 hours and 96.5% after 4 hours. This indicates that in the early stage of acid leaching, H ⁺ preferentially reacts with magnesite, and then gradually dissolves dolomite. Taking into account both efficiency and cost, the optimal acid leaching time is 4 hours.
2. The influence of acid leaching temperature
Temperature is a key factor affecting the rate of acid leaching reaction. When the temperature rises from 25 ℃ to 80 ℃, the dissolution rates of dolomite and magnesite increase from 71.3% and 85.6% to 96.5% and 99.1%, respectively. After the temperature exceeds 80 ℃, the removal rate and dissolution rate tend to stabilize, but the volatilization of hydrochloric acid intensifies and the operating environment deteriorates. Therefore, the optimal acid leaching temperature is 80 ℃.
3. The influence of solid-liquid ratio
The solid-liquid ratio directly affects the mass transfer efficiency of acid leaching reaction. The experimental results showed that when the solid-liquid ratio increased from 1:5 to 1:15g/mL, the dissolution rates of dolomite and magnesite increased from 82.1% and 90.5% to 96.5% and 99.1%, respectively. Further increasing the solid-liquid ratio to 1:30g/mL did not significantly improve the removal and dissolution rates, but the amount of acid solution used increased significantly, leading to an increase in costs. Therefore, the optimal solid-liquid ratio is 1:15g/mL.
4. The influence of stirring speed
The stirring speed affects the reaction rate by influencing the mass transfer efficiency. The experimental results showed that when the stirring speed increased from 100rpm to 300rpm, the dissolution rates of dolomite and magnesite increased from 90.3% and 95.6% to 96.5% and 99.1%, respectively. Further increasing the stirring speed to 500rpm did not significantly improve the removal and dissolution rates, but significantly increased energy consumption. Therefore, the optimal stirring speed is 300rpm.
Analysis of Acid Leaching Mechanism
During the acid leaching process, H ⁺ in hydrochloric acid reacts chemically with dolomite (CaMg (CO3) ₂) and magnesite (MgCO3), generating soluble chlorides and CO ₂ gas. The reaction equation is as follows:
Dolomite:
CaMg(CO3)2+4HCl→CaCl2+MgCl2+2CO2↑+2H2O
Magnesite ore:
MgCO3+2HCl→MgCl2+CO2↑+H2O
XRD analysis showed that the characteristic peaks of dolomite and magnesite disappeared in the solid product after acid leaching, and the diffraction intensity of talc (001) crystal plane significantly increased, indicating that the layered structure of talc was not destroyed. SEM observation shows that the surface of talc particles is smooth and the layered structure is clear after acid leaching, confirming the selective dissolution characteristics of talc and impurities by the acid leaching process.
Orthogonal experimental verification
To further optimize the process parameters, a four factor three-level orthogonal experiment (L ₉ (3 ⁴)) was designed, and the factor levels are shown in Table 2:
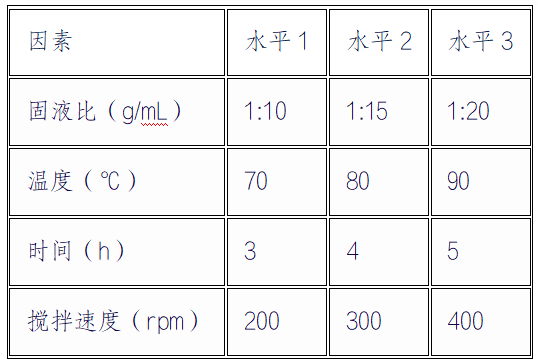
The experimental results indicate that the optimal combination is A ₂ B ₂ C ₂ D ₂ (solid-liquid ratio 1:15g/mL, temperature 80 ℃, time 4 hours, stirring speed 300rpm), which is consistent with the results of the single factor experiment.
4. Conclusion
Best process conditions:
Acid leaching time is 4 hours, temperature is 80 ℃, solid-liquid ratio is 1:15g/mL, and stirring speed is 300rpm. Under these conditions, the dissolution rates of dolomite and magnesite reached 96.5% and 99.1%, respectively, and the purity of talc increased to 89.3%.
Acid leaching mechanism:
H ⁺ reacts chemically with carbonate minerals to generate soluble chlorides, achieving impurity dissolution; The layered structure of talc is stable and has not been damaged by acid solution.
Application prospects:
The acid leaching process proposed in this study has the advantages of simple operation, low cost, and thorough removal of impurities. It is suitable for the resource utilization of talc solid waste and can provide technical support for the production of high-end talc products.
Prospect
Future research on Liaoning Xinda Talc Group Co., Ltd. can further explore the following directions:
Acid leaching solution recycling:
By using precipitation method to recover Mg ² ⁺ from acid leaching solution, magnesium hydroxide or magnesium oxide can be prepared to achieve closed-loop resource utilization.
Joint purification process:
By combining physical methods such as flotation and magnetic separation, the purity of talc can be further improved to meet the demand for high-end products such as ultrafine talc powder and high whiteness talc.
Environmental Impact Assessment:
Systematically evaluate the characteristics of wastewater and exhaust gas emissions from acid leaching processes, develop green treatment technologies, and reduce environmental risks.
References
1. Exploration and Experimental Study on Purification of Xinda Talc Tailings -2022.5.docx
2. Mineral processing and purification methods for talc
3. Research on the process of purifying low-grade talc by acid leaching method
4. Solid waste resource leaching++acid leaching


